Blog
How Specimen Tracking Software Improves Efficiency and Reduces the Chance for Diagnostic Errors
January 8, 2026
The Centers for Disease Control and Prevention estimates that approximately 14 billion laboratory tests are ordered each year. Combined with the fact that nearly 70 percent of medical decisions depend on laboratory results, the scale and clinical impact of diagnostics make one thing clear: accurate specimen tracking and efficient lab sample management are fundamental to patient care.
With this level of influence comes an equally significant responsibility. Molecular, clinical, and pathology organizations must rely on advanced lab sample management system software and disciplined processes to ensure accuracy, efficiency, and scalability across every stage of testing.
The stakes are high. The CDC estimates that 40,000 to 80,000 deaths annually are associated with preventable diagnostic errors. Alarmingly, research from Johns Hopkins suggests the problem may be even more severe, citing limitations in existing data collection and estimating that medical errors may be the third leading cause of death in the United States, contributing to more than 250,000 deaths each year.
Discover More: Top 10 Medical Laboratory Mistakes and How to Prevent Them from Happening in Your Lab
One of the most common (and preventable) causes of misdiagnosis is improper labeling and tracking of tissue samples and specimens. This is why a dependable, error-free pathology specimen tracking system is essential for laboratories of every size, starting with precise, standardized sample identification and consistent tracking practices throughout the entire workflow.
Get Insight: Reducing Risk in the Modern Laboratory: A Comprehensive Approach

What Sample Tracking Means in Modern Medical Laboratories
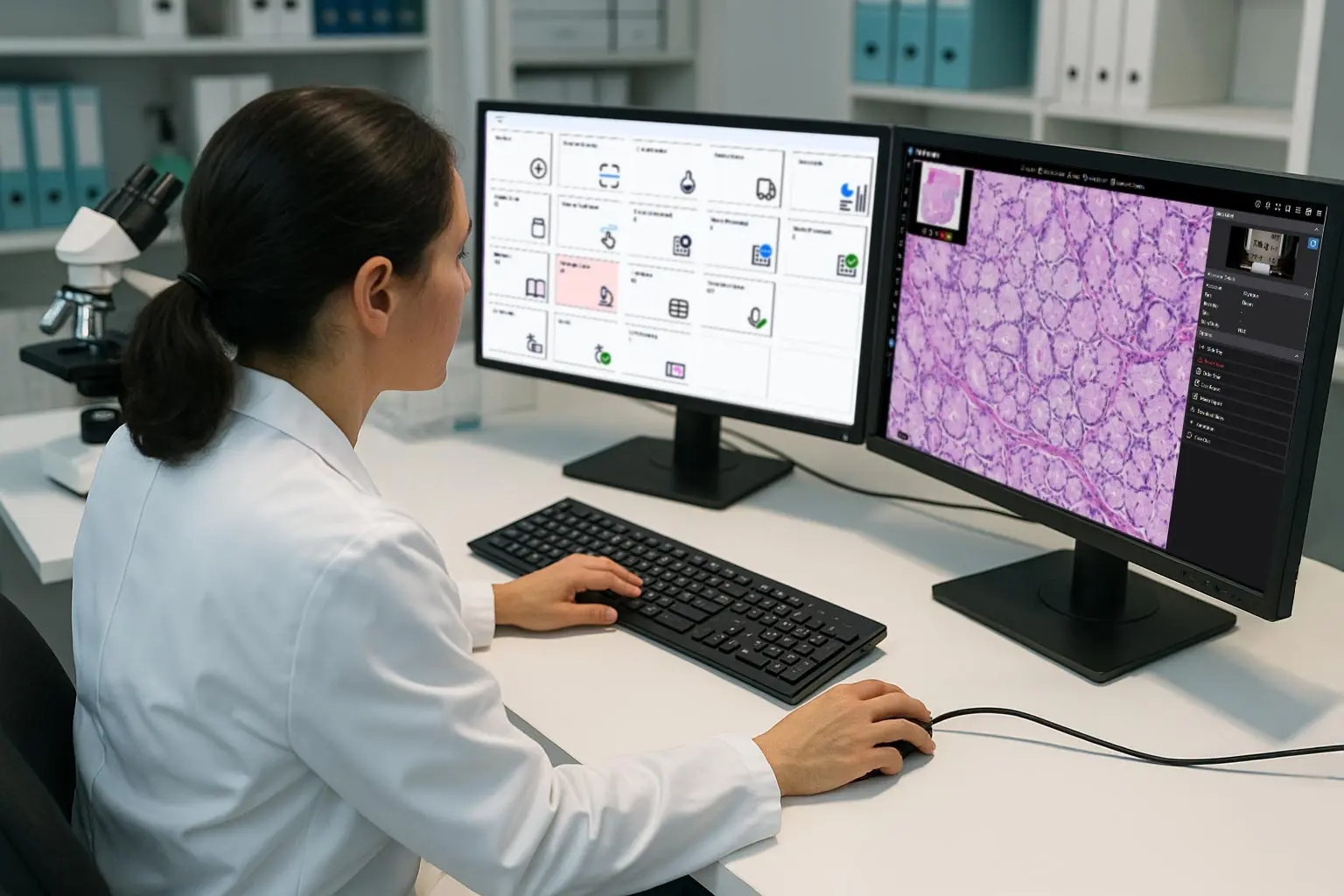
Sample tracking refers to the laboratory sample management process that ensures each patient specimen is accurately labeled, monitored, and moved through a laboratory information system (LIS) across the entire diagnostic testing lifecycle. From accessioning to final reporting, effective sample tracking maintains the chain of custody, prevents errors, and preserves full traceability at every step of the lab workflow.
Modern laboratory information systems, such as Ligolab’s all-in-one Medical LIS & Lab RCM Informatics Platform, combine advanced clinical and pathology reporting software with error-free sample tracking. These platforms provide complete visibility into medical lab workflows, enhance traceability, and support more efficient, accurate, and scalable laboratory operations.
Full Chain-of-Custody Visibility with Advanced LIS Sample Tracking
“With our all-in-one LIS system featuring advanced sample tracking, lab directors and managers always know who handled a specimen and where it moved throughout the workflow,” said Suren Avunjian, CEO of LigoLab. He added that the platform’s lab tracking system is designed to account for (and expose) workflow bottlenecks across the laboratory information system environment.
“We built LigoLab to closely reflect real-world laboratory operations, with full visibility into parent–child relationships as specimens, derivatives, and aliquots move through each stage of the lab,” Avunjian explained.
The LigoLab platform assigns a unique specimen identifier at the time an order is placed, ensuring specimen security across multiple workflows and processes. Each sample is then tracked through departments, racks, instruments, and testing steps, creating a complete chain of custody and a comprehensive, searchable audit trail.
Enterprise-Level Sample Traceability Across Every Laboratory Workflow
All specimens are fully traceable within the LIS system, including batch processing and send-out orders between facilities. When additional testing is requested, LigoLab’s pathology sample tracking solution enables laboratories to instantly access patient details and related historical testing data, eliminating delays and manual lookups. These advanced tracking capabilities are powered by LigoLab’s robust rule and automation engines.
By leveraging LigoLab’s enterprise-grade LIS software, laboratories enhance end-to-end workflow management while significantly reducing manual intervention and associated costs. The result is accurate, efficient sample tracking and more streamlined, scalable laboratory operations.
Discover More: How Best Practices and Advanced Laboratory Information System Technology Help Ensure Lab Workflow Management
Why Automated Sample Tracking is Essential for Patient Safety
Modern sample tracking pathology software applications, such as LigoLab’s sample tracking and handling module, reduce errors by ensuring every specimen is barcoded and assigned multiple identifiers. The module automatically generates a complete audit log that records where each specimen has traveled and which technician handled it at every step of the workflow.
Despite the availability of this technology, many laboratories still rely on manual, handwritten tracking logs. This approach not only consumes valuable technician time but also introduces unnecessary risk, increasing the likelihood of preventable errors.
Even the most accurate diagnosis loses its value if results are associated with the wrong patient. These mistakes can drive up healthcare costs, delay treatment, and, in some cases, lead to inappropriate care. Critically, a single tracking error can affect two patients: the one who is misdiagnosed and the one whose correct treatment is delayed.
For these reasons, protecting the integrity of patient information through reliable, automated sample tracking is a top priority for modern laboratories. It is a responsibility that should no longer depend on manual processes prone to avoidable and potentially serious errors.
Discover More: Paulette Lewis Talks About Specimen Tracking and Replacing Manual Processes with Automation
Why Specimen Labeling Errors Still Pose a Serious Risk
Not all laboratory errors stem from specimen mislabeling. Mistakes can occur for many reasons, including sample mishandling, slide contamination, or process breakdowns. That said, incorrect labeling remains one of the most significant contributors to patient misdiagnosis.
Patient identification errors in pathology specimens are estimated to occur in approximately 0.4 percent of cases, with about 0.1 percent directly attributed to mislabeling. While a 0.1 percent error rate may appear negligible, its impact becomes far more concerning when applied to the 14 billion laboratory tests performed each year.
“We help labs ensure that every specimen is visible and continuously tracked,” said Avunjian. “Each step and every specimen scan serves as an additional validation point, making sure nothing falls through the cracks.”
Avunjian explained that because LigoLab’s LIS system platform operates on real-time workflow queues, technicians can quickly identify specimens that remain in a queue longer than expected. Turnaround times are continuously monitored, and the system automatically highlights potential issues, such as changing the case color, to draw immediate attention to possible errors.
“It’s easy to blame mistakes on overburdened staff or management,” Avunjian added. “Most laboratories already go to great lengths to verify details. But when there are eight or more manual steps in a high-throughput testing process, human error becomes difficult to avoid. Automation and visibility are essential to reducing that risk.”
Get Insight: The Power of Integration - Unleashing the Potential of LigoLab's LIS System & Lab RCM Platform
.jpeg)
Rising Test Volumes and Staffing Shortages Increase Sample Tracking Risk
One of the biggest challenges tied to improper specimen collection and tracking is the sheer volume of testing performed by today’s medical laboratories. As test volumes continue to rise, so does the pressure to process specimens faster, often compressing timelines and increasing the risk of errors.
Compounding this challenge is the ongoing, industry-wide workforce shortage. Even before the recent pandemic, laboratories struggled to recruit and retain qualified personnel. COVID-19 intensified an already serious problem, transforming it into the staffing crisis organizations face today and further underscoring the need for efficient, automated sample tracking and laboratory workflow management systems.
Discover More: Laboratory Information System Software and Its Role in Overcoming Laboratory Staffing Challenges
Common Failure Points in Manual Sample Tracking Processes
When laboratories fill out, verify, and log patient details, there are multiple opportunities for error within the sample tracking process. Common risk points include:
- Misinterpreting faded labels or illegible handwriting
- Handling slides in batches, thereby increasing the risk of mismatching slides to the wrong tissue block
- Relabeling slides, creating additional opportunities for error
- Manually entering data into computer systems
- Using pre-printed labels that lack secondary identifiers, resulting in loose or misapplied labels
“The LigoLab approach is to generate specimen labels in real time, only when needed and at the exact point in the workflow,” said Avunjian. “This dramatically improves security and specimen tracking. Unfortunately, many laboratories still rely on pre-printed labels with legacy LIS systems, and that’s a recipe for failure.”
No matter how careful laboratory staff may be, human error is always a risk, especially when critical patient information must be captured repeatedly throughout the day. Without automated sample collection and tracking support, mistakes become not just possible, but inevitable.
Industry Insights: Just-In-Time vs. Batch Processing in the Medical Lab: Examining the Pros and Cons

Barcoding and Early Sample Identification Reduce Risk and Improve Efficiency
Uniquely identifying specimens early in the testing process and using barcoding combined with advanced pathology specimen tracking system functionality within LIS software dramatically improves efficiency while reducing errors. That’s why today’s leading laboratories rely on coded identification as a foundational element of their sample tracking workflows.
Many labs still depend on numeric identifiers, which often require samples to be relabeled as they move through the workflow, introducing unnecessary risk. Modern LIS lab platforms eliminate this vulnerability by embedding barcoding and scanning directly into sample-tracking LIS system functionality, allowing specimens to be verified at every step without manual relabeling.
“When barcoding is combined with LigoLab’s sample collection and tracking capabilities, laboratories can confirm the identity of every specimen across the entire clinical lab workflow,” said Avunjian. “If a mix-up occurs, the lab tracking system within our LIS software automatically alerts the lab. The event is logged so teams can investigate the root cause and prevent future errors.”
This approach ensures specimens are handled with clarity and consistency, eliminating confusion throughout the testing process. Each scan records who handled the sample and when, creating full traceability and a complete chain of custody.
“The most powerful pathology specimen tracking system solutions scale as laboratory volume and complexity grow,” Avunjian added. “The best LIS software enables highly granular tracking at every stage of testing, improving specimen management accuracy and significantly reducing errors.”
Discover More: Can Your Laboratory Information System Support the Best LIS System Technology?

The Rise of Advanced Scanning and RFID in LIS Systems
Most laboratories still rely on handheld barcode scanners to track specimens. However, clinical and pathology LIS systems with built-in scanning and automation capabilities are gaining traction. One emerging example is the use of Radio Frequency Identification (RFID), enabled labels, which can be recognized automatically by LigoLab’s LIS system platform without requiring individual scans.
“Typically, this technology is applied at the rack level,” said Avunjian. “Our platform is aware of every specimen within a rack, so as the rack moves throughout the laboratory, the LIS system automatically records and tracks those movements.”
While RFID technology is already available, adoption within pathology laboratories has been limited to date. That is expected to change as more laboratory information system vendors follow LigoLab’s lead and develop clinical and pathology lab software that supports more advanced, automated approaches to specimen tracking and workflow visibility.
Discover More: What You Need to Know Before Contracting with a Laboratory Information System (LIS) Company
A Unified Informatics Platform for Clinical, Molecular, and Anatomic Pathology
The molecular, clinical, and anatomic pathology laboratory suites sit at the core of LigoLab's all-in-one Medical LIS & Lab RCM Informatics Platform. These suites function as centralized data hubs for all cases, supporting rapid order entry, label and barcode generation, comprehensive specimen tracking, document scanning, touch-enabled grossing workflows, electronic histology, efficient result entry and sign-out, image acquisition, and direct digital integration with microscopes and whole slide scanners.
Additional capabilities include advanced reporting, quality control, integration with prior cytology results, automated reflex testing, and more. Designed for both general and specialized clinical laboratories and pathology practices of any size, the LigoLab platform integrates seamlessly with existing hospital management systems, delivering flexibility, scalability, and enterprise-grade performance.
Industry Insights: The Competitive Edge in Laboratory Outreach - Enhancing Value Beyond Price
An Award-Winning, End-to-End LIS System Platform for Modern Laboratories
LigoLab is an award-winning developer of innovative, end-to-end laboratory information system software, supporting hundreds of laboratory facilities nationwide.
The enterprise-grade LigoLab Informatics Platform delivers a fully integrated solution with dedicated modules for anatomic pathology, clinical laboratory, and molecular diagnostics workflows. Additionally, it has embedded laboratory revenue cycle management and direct-to-consumer lab testing modules, all unified on a single, powerful platform that supports every role, department, and case.
Widely recognized as a leader across the laboratory industry, LigoLab empowers labs to deliver better patient care, differentiate in competitive markets, scale with confidence, and operate with greater compliance, efficiency, and profitability.
Ready to Unify Your Lab on One Powerful Platform?
Discover how the LigoLab Informatics Platform enables lab customers to scale confidently, improve compliance, and drive profitability.
Act Now: Speak with a LigoLab Product Specialist!