Industry Insights
Best LIS Systems in 2026: Top Laboratory Information Systems Compared for Clinical, Pathology, and Outreach Labs
January 5, 2026
What is an LIS System?
A laboratory information system (LIS) is software that manages the end-to-end lifecycle of laboratory diagnostics, from order intake and specimen tracking to testing workflows, result reporting, quality control, compliance, and increasingly, lab billing and operational analytics.
Modern LIS software platforms extend beyond basic data management. They function as systems of action, coordinating people, instruments, workflows, and financial processes in real time to improve efficiency, reduce errors, and support scalable laboratory operations.
Why the Definition of the “Best LIS System” Has Changed, and What Modern Laboratories Now Require
Modern laboratories rarely fail because of a single catastrophic breakdown. Instead, inefficiency accumulates quietly through dozens of small handoffs: incomplete order data, manual worksheets, faxed add-ons, misplaced specimens, interface queues or worksheets without clear ownership, lab billing edits discovered too late, or pathology cases delayed because images, metadata, and financial information live in separate systems.
These operational gaps compound at scale, slowing turnaround times, frustrating providers, straining staff, and quietly eroding margins. Over time, they impose an artificial ceiling on a laboratory’s ability to grow.
As a result, the definition of the “best LIS system” has fundamentally changed.
Today, laboratories no longer evaluate LIS software solely on accessioning and result reporting capabilities. The most effective laboratory information systems are designed to coordinate the entire diagnostic and financial lifecycle, connecting technical workflows, outreach operations, and lab revenue cycle management (lab RCM) processes into a single operating environment.
The platforms that stand out are those that:
- Orchestrate end-to-end laboratory workflows across departments and locations
- Support clinical, anatomic pathology, molecular, and specialty diagnostics without fragmentation
- Apply configurable rules and automation to reduce manual intervention
- Unify operational and financial data to manage throughput and cash flow together
- Prevent revenue leakage by enforcing lab billing readiness upstream
- Integrate seamlessly with instruments, EHRs, and reference laboratories
- Scale without relying on bolt-on modules that create new silos
This guide brings a market-based comparison of the leading LIS systems in use today, examining where each platform excels, where tradeoffs exist, and why unified, all-in-one architectures are increasingly replacing modular LIS models.
Discover More: Four Game-Changing Business Strategies to Improve Laboratory Processes
How to Evaluate LIS Platforms Beyond Basic Demos and Core Functionality
Labs evaluating LIS platforms often request side-by-side demos of workflow automation, exception handling, and billing readiness. While most modern LIS systems can handle core functions like accessioning, result reporting, and basic interfaces, the real differentiators emerge under pressure.
Laboratories should evaluate the depth of the system, how much autonomy internal teams have to configure and evolve workflows on their own, and how effectively the platform manages real-world exceptions rather than only ideal, linear workflows.
Act Now: Contact LigoLab to Schedule a Personalized Demo

A Summary Comparison of Today’s Leading LIS Systems
Let’s start with an overview of the top LIS systems currently in widespread use, highlighting core strengths and primary tradeoffs.
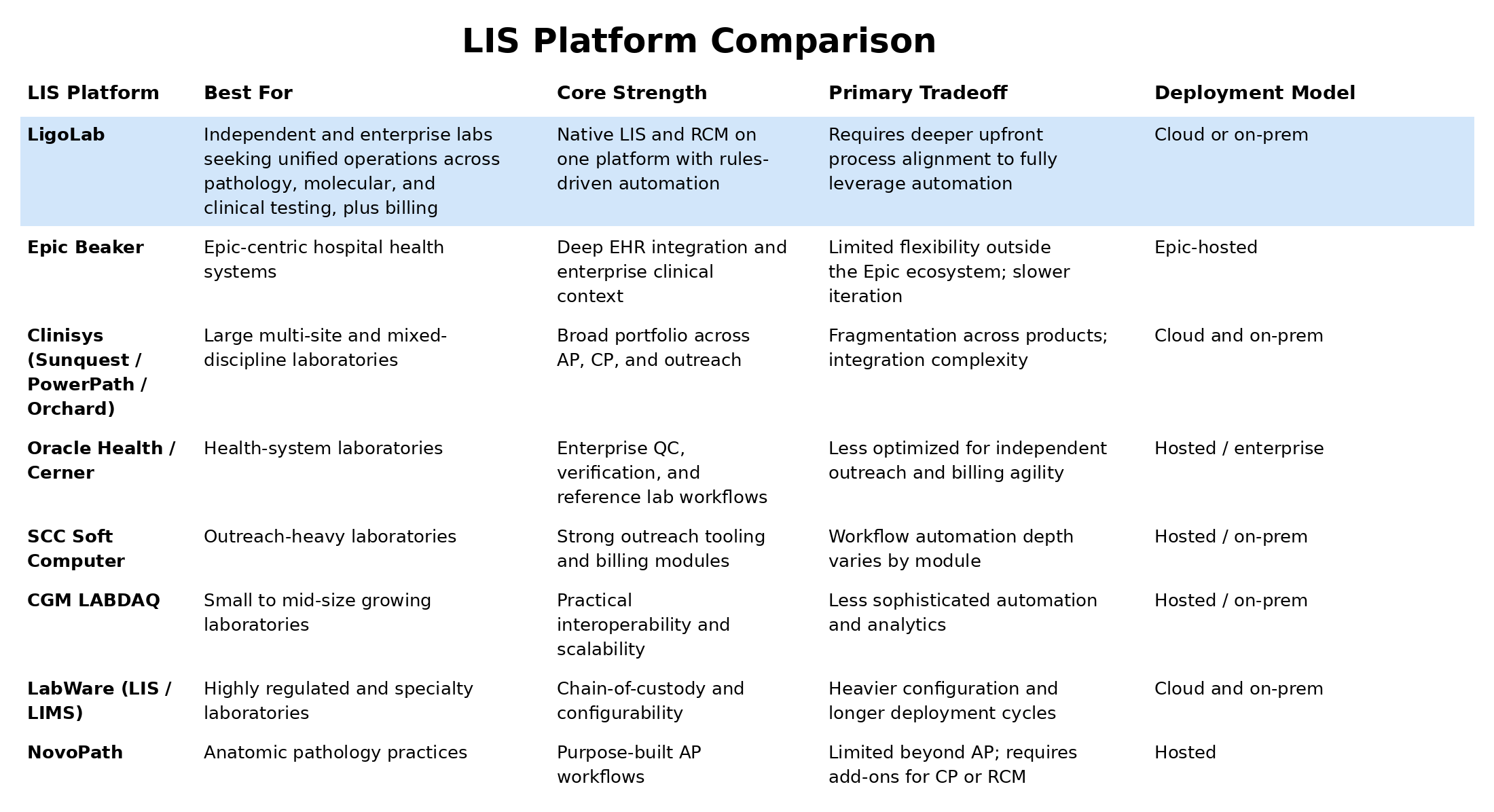
What Most LIS Systems Get Right (and Where the Differences Begin)
Across clinical laboratories, pathology practices, and multi-site diagnostic organizations, the most successful LIS system implementations consistently share a common set of capabilities.
The best LIS systems today are characterized by:
- Rules-based workflow automation that governs routing, autoverification, exception handling, and task prioritization rather than relying on manual decision-making.
- True specimen-to-result traceability, including complete audit trails, chain-of-custody visibility, and real-time status transparency.
- High-throughput instrument and middleware connectivity with resilient, bidirectional interfaces and managed queues.
- Configurable worklists and dashboards that surface bottlenecks by role, department, and priority.
- Outreach and interoperability infrastructure supporting EHR integration, portals, ordering control, and multi-site operations.
- Built-in quality and compliance controls aligned with regulatory and accreditation requirements.
- Lab revenue cycle management intelligence that’s embedded upstream, ensuring demographic accuracy, coding readiness, and claim viability before results are finalized.
While many LIS software platforms excel in individual domains, such as hospital integration or anatomic pathology, only a small number unify operational workflows, outreach, and revenue cycle processes within a single, cohesive informatics platform.
Discover More: Is Your Laboratory Information System Able to Support the Latest LIS System Technology?
Top LIS System Platforms: Strengths, Tradeoffs, and the Rise of All-in-One Architectures
The analysis below examines the leading LIS system platforms on the market; what they do exceptionally well, where tradeoffs exist, and why all-in-one architectures, particularly those that unify LIS and RCM workflows, are emerging as the defining competitive advantage for modern laboratories.
1) LigoLab’s All-in-One LIS & RCM Informatics Platform
Best-in-class for laboratories seeking a unified LIS + RCM architecture: LigoLab’s differentiator is a native, unified LIS + RCM architecture. The LIS system and lab RCM modules live on one unified informatics platform that ties technical operations directly to lab billing workflow, so errors are prevented upstream instead of “fixed later.” LigoLab is used by multi-facility pathology groups processing millions of accessions annually.
On-Demand Webinar: From Manual to Magical - Automation Strategies for Multi-Facility AP & Clinical Labs
Advanced Functions
- Cloud-ready deployment and flexibility: LigoLab supports cloud-based deployment and traditional on-premises server installations, providing laboratories the flexibility to choose the environment that best fits their infrastructure, security requirements, and operational goals.
- Support for diverse diagnostic disciplines: Rather than forcing laboratories to manage separate LIS instances or stitched-together modules, LigoLab enables clinical, anatomic pathology, and molecular diagnostics workflows to coexist and scale on a single, highly configurable informatics platform.
- Rules-driven automation across the entire lab workflow: A sophisticated automation engine triggers multi-step actions, prioritization logic, routing, and exception handling across departments. By automating full workflows, not just individual tasks, the platform reduces manual touchpoints per specimen and enables laboratories to scale volume without adding headcount.
- End-to-end specimen and case lifecycle control: Shared workflow queues, real-time status visibility, and complete audit trails ensure every specimen, case, and financial event is managed within a single system of record (one source of truth). This unified control model eliminates departmental friction while improving turnaround times, accountability, and operational consistency.
- Integrated laboratory RCM automation: Built-in demographic review, eligibility verification, insurance discovery, intelligent workflow queues, and automated CPT/ICD coding proactively reduce denials and rework. Revenue protection is embedded directly into LIS system workflows, accelerating cash flow and improving lab billing accuracy.
- Unified operational and financial intelligence: Real-time dashboards connect throughput, turnaround time, staffing efficiency, and revenue performance in one view. This unified intelligence allows laboratory leaders to manage operations and margins together, rather than relying on disconnected reports or retrospective analysis.
Bottlenecks it Eliminates
- Manual exception chasing across departments: Missing demographics, coverage issues, and medical-necessity gaps are prevented upstream through rules-driven enforcement and shared queues, stopping exceptions before they cascade downstream.
- “Billing after the fact” revenue delays: By embedding demographic review, eligibility checks, insurance discovery, and coding logic directly into LIS system workflows, cases remain billing-ready at every stage of processing, reducing delays and denial risk.
- Departmental silos and handoff friction: Accessioning, technical benches, client services, and laboratory billing departments all operate within a single system of action, utilizing shared rules, queues, and real-time visibility to streamline handoffs and improve turnaround times.
- Scaling constraints as volume grows: Automation reduces human touchpoints per specimen while supporting multi-facility operations. Shared, real-time workflow queues provide all teams with full visibility into specimen status, workload, and downstream dependencies across all testing disciplines, enabling laboratories to increase test volume and complexity without proportional staffing increases.
Bottom line: Unlike traditional LIS systems that rely on disconnected modules or third-party laboratory billing platforms, LigoLab’s informatics platform unifies clinical operations, outreach, automation, and lab revenue cycle management into a single, rules-driven system, eliminating silos, reducing errors upstream, and enabling laboratories to scale efficiently without rebuilding their technology stack.
2) Epic Beaker LIS
Why it’s considered top-tier: Beaker is most often chosen by health systems that have standardized on Epic systems and prioritize tight integration with the enterprise electronic health record (EHR). Its primary strength lies in providing rich clinical context and unified patient records across the organization.
Advanced Functions
- Delivers a single, enterprise-wide clinical context when Epic functions as the core EHR, minimizing system switching and improving clinical data access for both anatomic and clinical pathology workflows.
- Provides modular anatomic pathology and clinical pathology components, enabling alignment with standardized, enterprise-wide clinical workflows.
Bottlenecks it Eliminates
- Delays in clinical data access for pathologists and laboratory teams working within an Epic-centric environment.
- Fragmented ordering and results distribution by consolidating workflows within a single, enterprise-wide EHR hub.
Tradeoff to watch: Beaker tends to shine most when your enterprise is already committed to Epic. For independent labs or multi-EHR outreach businesses, best-of-breed connectivity and specialized lab billing automation can become deciding factors.
Constraint: Customization and iteration speed may be limited by enterprise governance and Epic release cycles.
3) Clinisys LIS (Sunquest, PowerPath, Orchard)
Why it’s considered enterprise-grade: Clinisys has one of the broadest portfolios in laboratory informatics, spanning clinical pathology, anatomic pathology diagnostics, outreach/networking, and cloud-based modernization initiatives. Clinisys positions its cloud offerings around scalability, analytics, and reducing data silos.
Advanced Functions
- Orchard Enterprise delivers rule sequencing, autoverification, analytics, KPI tracking, and workflow optimization designed to improve operational efficiency.
- PowerPath (Sunquest PowerPath) is an anatomic pathology–focused LIS system with greater diagnostic flexibility and workflow depth than typical EHR-based pathology modules.
- Atlas outreach and multi-lab networking capabilities harmonize data across multiple LIS system environments, streamline order processing and results delivery, and enable test routing across distributed laboratory networks.
- Ongoing platform enhancements emphasize new functionality, modernization initiatives, and expanded support tooling across the portfolio.
Bottlenecks it Eliminates
- Multi-site operational complexity and “siloed LIS islands” through networking and data harmonization capabilities.
- Manual review workload by applying rule sequencing and autoverification to reduce repetitive human intervention.
- Operational friction for outreach, particularly around ordering, catalog synchronization, and results delivery, across heterogeneous laboratory environments.
Constraint: Portfolio breadth can introduce operational complexity, requiring integration and coordination across multiple products.
4) Oracle Health / Cerner PathNet LIS
Why it’s considered top-tier: Oracle Health positions its laboratory offerings around workflow streamlining, verification, reference lab integration, QC, and capturing billable events, often in health-system contexts.
Advanced Functions
- Configurable verification workflows with site-specific reference ranges and built-in turnaround-time monitoring.
- Integrated quality control processes, secondary result review, and seamless reference laboratory connectivity.
- Embedded capture of billable events within clinical workflows to support accurate and complete revenue recognition.
Bottlenecks it Eliminates
- Variability in result verification processes and inconsistent quality control enforcement.
- Reference laboratory coordination friction within complex, enterprise laboratory environments.
Constraint: Best suited for health-system environments; independent outreach and rapid lab billing innovation can be more constrained.
5) SCC Soft Computer LIS
Why it’s considered top-tier: SCC emphasizes a comprehensive LIS ecosystem with outreach and RCM capabilities, including web-based reporting and dedicated lab billing/accounts receivable tooling.
Advanced Functions
- Comprehensive outreach suite that includes web-based ordering and results delivery, along with integrated lab billing and accounts receivable modules designed to support profitable outreach programs.
- Lab revenue cycle management solutions focused on streamlining billing workflows, improving collections efficiency, and strengthening overall financial performance.
- Specialized, integrated add-on capabilities, such as biobank management tools, are embedded directly within the LIS environment.
Bottlenecks it Eliminates
- Customer service overload related to outreach, including excessive status inquiries and manual result chasing.
- Fragmented billing and collections workflows are addressed by providing a more structured, centralized approach to accounts receivable and billing operations.
Constraint: Advanced automation and cross-department orchestration may require additional configuration or external tools.
6) CGM LABDAQ LIS
Why it’s considered top-tier: CGM LABDAQ is commonly selected by laboratories that need a practical, scalable LIS system with strong connectivity to EHR/practice management and reference labs.
Advanced Functions
- Robust connectivity and interoperability, including interfaces for orders, demographics, results, laboratory billing data, and seamless reference laboratory integration.
- A scalable architecture that supports growth through the addition of users, sites, interfaces, and optional modules as laboratory needs evolve.
Bottlenecks it Eliminates
- Manual exchange of orders and results with external providers and reference laboratories through automated, bidirectional interfaces.
- Growth limitations associated with outgrowing entry-level or “starter” LIS systems are addressed by enabling incremental expansion of functionality and connectivity over time.
Constraint: Designed for scalability, but lacks the depth of rules-driven automation found in enterprise-grade platforms.
7) LabWare LIS and LIMS Platforms
Why it’s considered top-tier: While often categorized as laboratory information management systems (LIMS), LabWare positions its solutions for high configurability, chain-of-custody rigor, and automation in regulated environments; its Clinical Health releases also highlight HL7 integration, dashboards, and capabilities related to lab billing.
Advanced Functions
- Comprehensive chain-of-custody management combined with work assignment and inventory/storage location tracking to support highly regulated laboratory environments.
- Clinical Health–focused capabilities emphasizing system integration and operational automation across laboratory workflows.
Bottlenecks it Eliminates
- Compliance-intensive tracking gaps in environments that require detailed specimen governance and traceability.
- Manual coordination of complex testing protocols across personnel, instruments, and laboratory sites.
Constraint: High configurability comes with longer implementation timelines and greater reliance on specialized resources.
8) NovoPath LIS for Anatomic Pathology
Why it’s considered top-tier: NovoPath is purpose-built for anatomic pathology and is widely recognized for its focus on streamlining pathology workflows, automation, and detailed diagnostic reporting.
Advanced Functions
- Anatomic pathology–centric workflow automation and reporting designed to support the full pathology lifecycle, from accessioning through final diagnosis.
Bottlenecks it Eliminates
- Manual anatomic pathology handoffs, from grossing and histology to sign-out and reporting, by replacing fragmented steps with a more cohesive, purpose-built AP workflow design.
Constraint: Purpose-built for anatomic pathology, with limited native support for clinical diagnostics or revenue cycle workflows.
Discover More: Why “The Safe Move” to a New Version of Legacy LIS Software Is the Wrong Move for Medical Labs

Laboratory Bottlenecks Eliminated by the Best LIS Systems
Across these leaders, the “big wins” map to a few recurring bottleneck categories:
1) Accessioning and Pre-Analytic Chaos
Modern solution: Structured order intake, rules-based validation, automation to catch missing fields, and tight traceability.
Best-in-class impact: Fewer recollects, fewer call-backs, fewer lost specimens.
2) Bench Throughput and Result Verification Delays
Modern solution: Logic for autoverification, exception-based work queues, instrument connectivity, and real-time operational dashboards.
Best-in-class impact: Higher volume per FTE, fewer manual reviews, faster TAT.
3) Outreach Growth Bottlenecks
Modern solution: Ordering/catalog governance, client portals, multi-site routing, and interoperability layers that reduce friction between disparate systems.
Best-in-class impact: Fewer missing orders, cleaner interface connectivity, happier providers, scalable outreach.
4) Revenue Leakage and Denial Churn
Modern solution: Front-end insurance/eligibility controls, coding support, claim workflow automation, and auditable work queues.
Best-in-class impact: Fewer rejections, fewer denials, faster cash, less manual billing rework.
Most LIS systems can help labs move specimens faster and more accurately, but far fewer can also systematically prevent laboratory billing errors by design because billing is external or downstream. An advanced LIS software platform with a unified LIS+RCM architecture directly targets this industry-wide weak point.
Why All-in-One LIS Platforms Are Replacing Modular LIS Models
Historically, laboratories adopted modular technology stacks: one system for accessioning, another for pathology, a separate outreach portal, and an external laboratory billing software platform downstream. While this approach offered short-term flexibility, it introduced long-term operational risk.
As test complexity, payer requirements, and outreach volume increase, fragmented systems create:
- Data latency between operational and financial workflows
- Manual reconciliation across departments
- Ambiguity around ownership of exceptions
- Higher integration maintenance costs
- Delayed visibility into revenue risk
All-in-one LIS software platforms address these issues by treating the laboratory as a single operating environment rather than a collection of disconnected tools.
By unifying technical workflows, outreach operations, and revenue cycle processes on one software infrastructure, these platforms enable shared rules, shared queues, and shared data models. Errors are prevented upstream, accountability is clearly defined, and operational efficiency and financial performance improve together.
For laboratories planning growth over the next three to five years, this architectural shift is becoming a prerequisite rather than a differentiator.
On-Demand Webinar: From Systems of Record to Systems of Action
How to Choose the Best LIS System for Your Laboratory
The most revealing LIS system evaluations do not focus on feature checklists. They focus on how a system performs under real operational pressure.
Most modern LIS software platforms can support foundational functions such as accessioning, specimen tracking, result reporting, and basic interfaces. These capabilities are now table stakes. The true differences emerge when laboratories encounter variability: missing or incomplete orders, complex payer requirements, multi-site coordination, shifting priorities, and downstream revenue risk.
When evaluating a laboratory information system, organizations should ask LIS vendors to demonstrate how their systems handle real-world scenarios, including:
- Preventing missing or invalid order data from becoming laboratory billing problems
- Capturing, routing, and resolving exceptions such as missing specimens or operational failures
- Managing cross-department handoffs without manual coordination or workarounds
- Responding to provider status inquiries without internal escalation
- Identifying revenue risk before claims are submitted
- Distributing cases dynamically using rules and automation rather than static, generic workflows
Equally important is who controls change. Laboratories should assess how much autonomy internal teams have to configure rules, evolve workflows, and adjust case distribution as conditions change, without relying on vendor intervention or professional services.
While many LIS platforms can function as systems of record, laboratories focused on scale, automation, and margin protection increasingly require systems of action. A unified LIS and lab revenue cycle management platform, built on a single software infrastructure, provides the visibility, control, and resilience needed to operate efficiently as volume and complexity grow.
White Paper: What To Do When Your Laboratory Information System Is Sunsetting
Evaluate LigoLab Against Other Top-Tier LIS System Platforms
To see how this approach compares in practice, contact a LigoLab product specialist to schedule a personalized demonstration and learn how LigoLab stacks up against other top-tier LIS systems, across workflow automation, laboratory revenue cycle management performance, and enterprise-scale pathology and clinical laboratory management.
Act Now: Contact a LigoLab Product Specialist Today!
LIS Demo Checklist: Questions Every Laboratory Should Ask
The most effective laboratory information system demos move beyond feature walkthroughs and focus on how the LIS system behaves under real operational pressure. Use the questions below to evaluate depth, flexibility, and long-term fit.
1. Workflow Depth and Automation
- Show how workflows are configured. Can rules be created, modified, and deployed by internal teams without vendor intervention?
- Demonstrate a multi-step workflow that spans accessioning, testing, review, and lab billing readiness. How are dependencies enforced?
- How does the LIS system handle non-linear workflows when steps occur out of sequence?
2. Exception Handling (Not the Happy Path)
- Demonstrate what happens when an order is missing demographics, insurance, or required clinical information.
- Show how exceptions are surfaced, prioritized, and routed. Are they visible in role-specific queues or buried in generic task lists?
- How does the LIS system prevent exceptions from cascading downstream into delays or billing issues?
3. Case Distribution and Workload Management
- Show how cases are distributed across benches, staff, or sites. Is the distribution static or rules-driven?
- Can routing account for priority, turnaround time, skill set, or capacity?
- How does the LIS system rebalance work when volume spikes or staff availability changes?
4. Autonomy and Change Management
- Who controls workflow changes: the lab or the vendor?
- How long does it take to implement a new rule, report changes, workflow, or routing change?
- Can changes be tested safely before going live?
5. Cross-Department and Multi-Site Visibility
- Demonstrate end-to-end visibility of a specimen or case across departments.
- How are handoffs managed without emails, spreadsheets, or manual follow-ups?
- Can leadership see operational and financial status in real time across sites?
6. Revenue Protection and Lab Billing Readiness
- Show how the LIS system validates lab billing readiness before results are finalized.
- How are payer rules, eligibility checks, and coding dependencies enforced upstream?
- Can the LIS system identify revenue risk before a claim is submitted?
7. Provider and Client Experience
- Demonstrate how staff respond to provider status inquiries. Is information immediately visible?
- How are outreach orders, add-ons, and corrections handled without disrupting workflow?
- What tools reduce inbound calls rather than shift the burden to staff?
8. Scalability and Future Readiness
- What happens when test volume doubles or new disciplines are added?
- Can the same workflows scale across multiple facilities without duplication?
- How does the platform avoid adding bolt-on modules as complexity increases?
Final Question to Ask Every Vendor
- If our workflows, payer mix, or volume change significantly over the next two years, how much of that adaptation can we manage ourselves versus needing vendor services?
The best laboratory information system software will demonstrate not only that it can process specimens, but that it can adapt, prevent exceptions, and support growth without increasing operational friction.
Discover More: Questions All Pathology Labs Should Ask When Evaluating LIS Software and Support







