Blog
How Molecular Test Orders are Accessioned in Lab with LigoLab's LIS System
July 10, 2025
With a return to normalcy, the molecular diagnostics laboratories that invested in PCR testing technology and infrastructure to meet the demands of the COVID-19 pandemic can now pivot, expand their test menus, and provide other forms of molecular LIS testing for their clients.
With modern and flexible molecular lab testing software, such as laboratory information systems (LIS software) serving as the centerpiece, these labs can now configure new analytical workflows and repurpose capacity that was once dedicated to high-throughput COVID testing.
Instead of SARS-CoV-2, they can now turn their attention and focus on other forms of infectious disease. This includes testing for sexually transmitted infections, urinary tract infections, and fungal, respiratory, and viral pathogens.
Discover More: A Scalable Solution for Molecular LIS Testing Labs
The all-in-one LigoLab Medical LIS & Lab RCM Informatics Platform is a perfect example of a comprehensive and highly configurable LIS laboratory information system. It comprises powerful modules supporting all testing disciplines, including anatomic pathology, clinical laboratory, and molecular diagnostics. Additionally, LigoLab’s enterprise pathology lab management platform also includes modules for laboratory revenue cycle management (lab RCM) and direct-to-consumer lab testing (TestDirectly patient outreach software).
Every module embedded within LigoLab’s medical LIS software shares the same database and infrastructure. There are no data silos with this combined lab information system and lab billing platform. It’s tailor-made for independent clinical laboratories and anatomic pathology groups interested in maximizing clinical lab workflow automation, expanding services, and scaling operations while gaining and retaining customers.
Discover More: The Future of LIS Systems and Personalized Medicine
What elements of the molecular lab software module help the LigoLab platform stand out when compared to the competition for the best laboratory information system software?
Let’s start by taking a look at how molecular lab software specimens are accessioned in a molecular LIS software environment.
On-Demand Webinar: Tour LigoLab’s Clinical Laboratory Module!
Accessioning a Test Order in the Molecular LIS Module
The Molecular LIS module is designed to support every role and department in the lab with flexible, role-based permissions. In the image below, the user is logged in as an Administrator, granting full access to all workflow queues, modules, and departments.
For other users, access can be easily customized, ensuring they only see the specific workflow queues relevant to their responsibilities as specimens move from accessioning through processing.
.webp)
You can also see in the same image that the user is in the Ordering Module. To start accessioning a manual order, the user clicks on the New Order workflow queue button.
Also, take note that there is a Remote Orders workflow queue as well. This is where orders from a client’s electronic health record (EHR) interface or LigoLab Web Connect, LigoLab’s provider portal, first enter the lab with pre-populated fields. The Remote Orders workflow queue also supports incoming orders from TestDirectly, LigoLab’s direct-to-consumer lab testing portal.
Discover More: Highlighting the Versatility of the TestDirectly Direct-to-Consumer Lab Testing Portal
Adding Details to a New Lab Test Order in the Molecular LIS Module
Now let’s continue with our look at a manual order in the New Order workflow.
The next step for the accessioned items in the lab order is to add the necessary information to the Order Details window manually.
In the screenshot below, you’ll notice three tabs, General, Patient, and Billing, each containing required fields that must be completed before the order can be saved. These fields are highlighted in red and include Client, Ordering Provider, Patient Last Name, and Patient First Name. The Payment Source field can also be set as required, as demonstrated in this example environment.
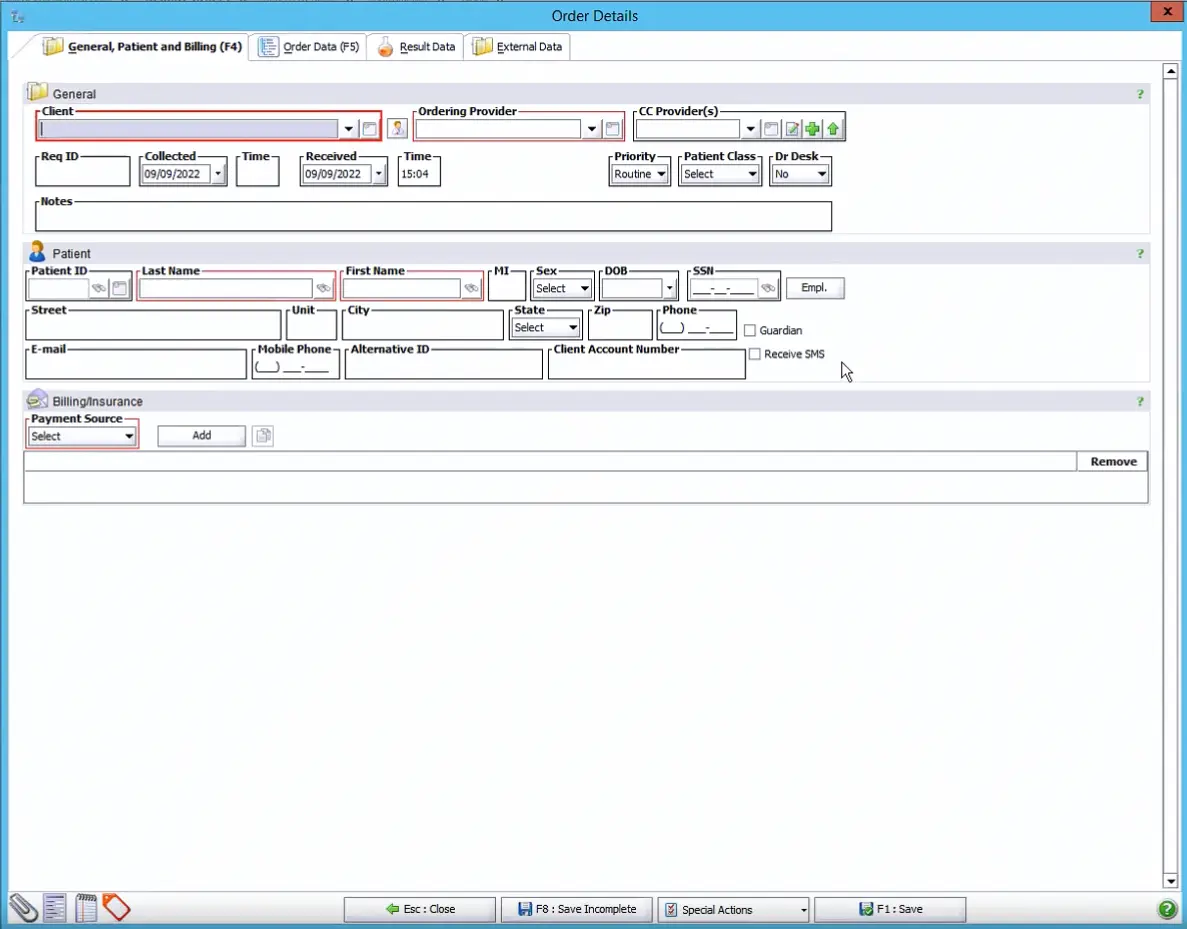
By entering a combination of the patient’s name and date of birth, the molecular lab software automatically checks for an existing patient profile within the LIS system. If a match is found, a pop-up window will appear, allowing the user to link the current order to the existing profile.
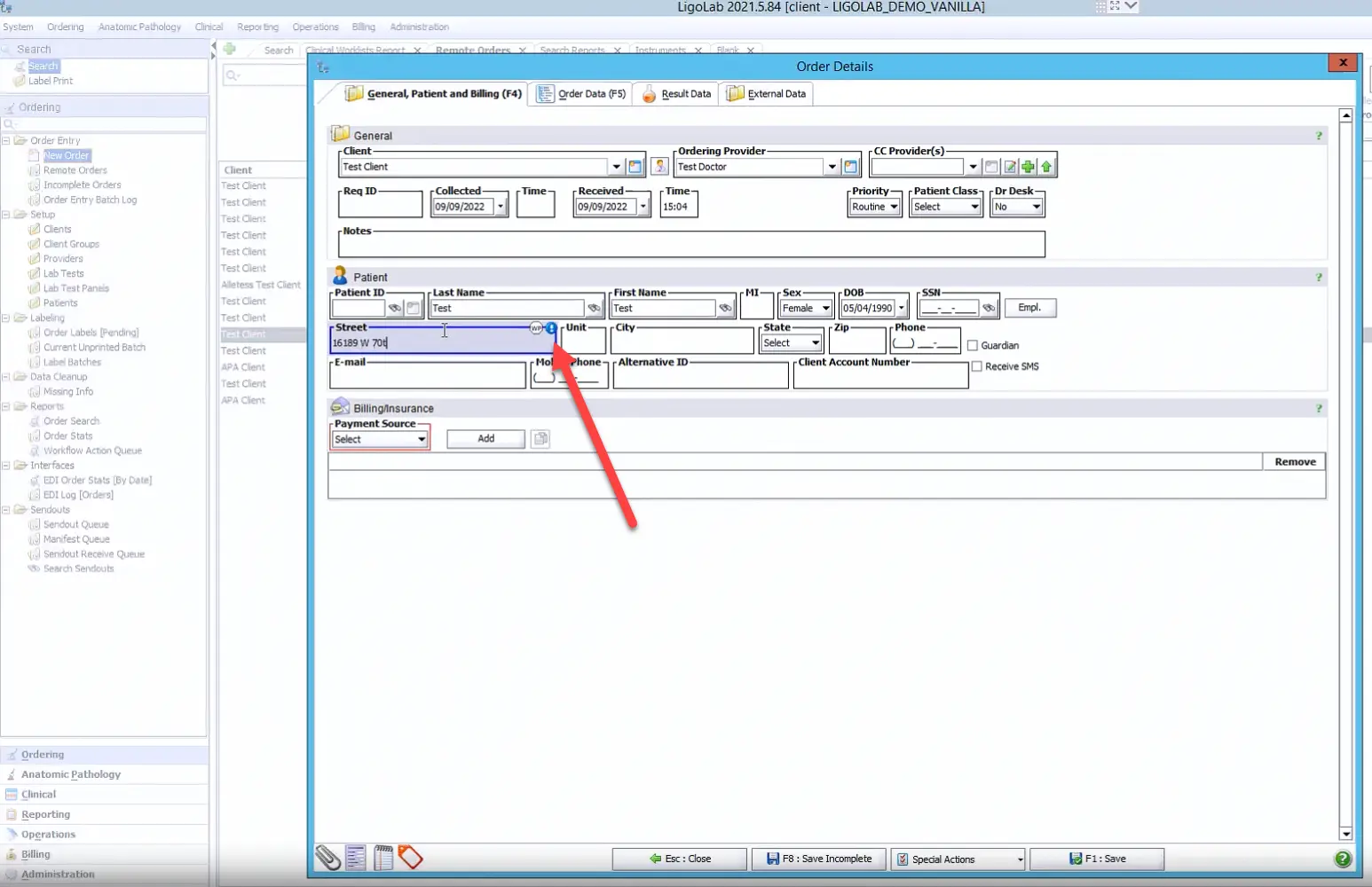
Additionally, the laboratory information system supports real-time address validation through integrations with the United States Postal Service and Whitepages. Users can trigger this feature by clicking the blue icon to the right of the Street field, or the adjacent WP icon, as shown below.
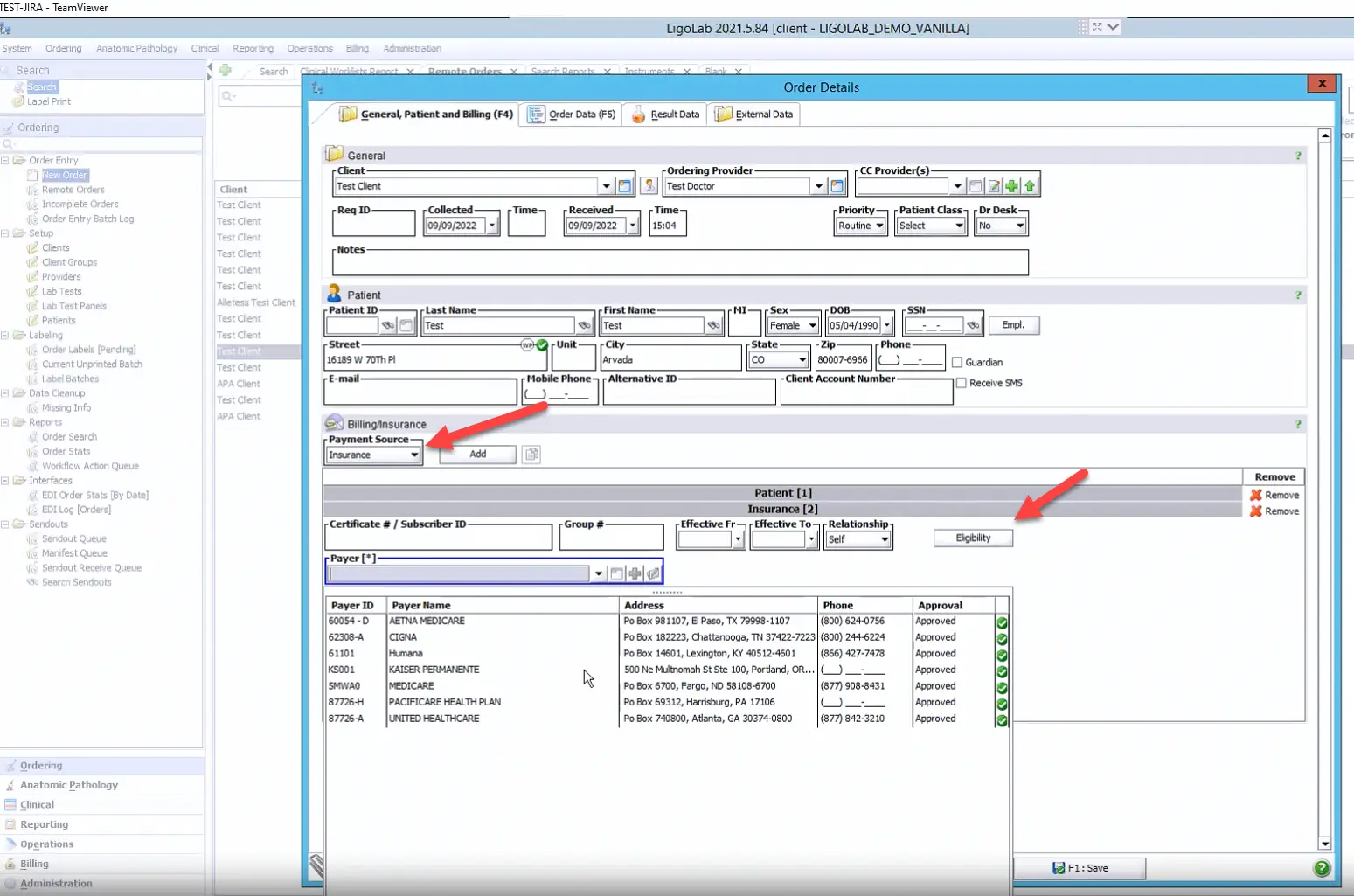
For LigoLab customers who have also licensed the laboratory billing/lab RCM module, real-time insurance eligibility checks and insurance discovery validation are also available. Both represent a significant advantage for the lab as they greatly increase the clean claim ratio and dramatically lower the risk of compliance liability.
Next, the user adds the Payment Source. In the example below, insurance has been selected from the Payment Source drop-down menu. Please note that the Insurance Eligibility button has also been highlighted.
Discover More: Comparing LigoLab Informatics Platform with Legacy Laboratory Information System Software
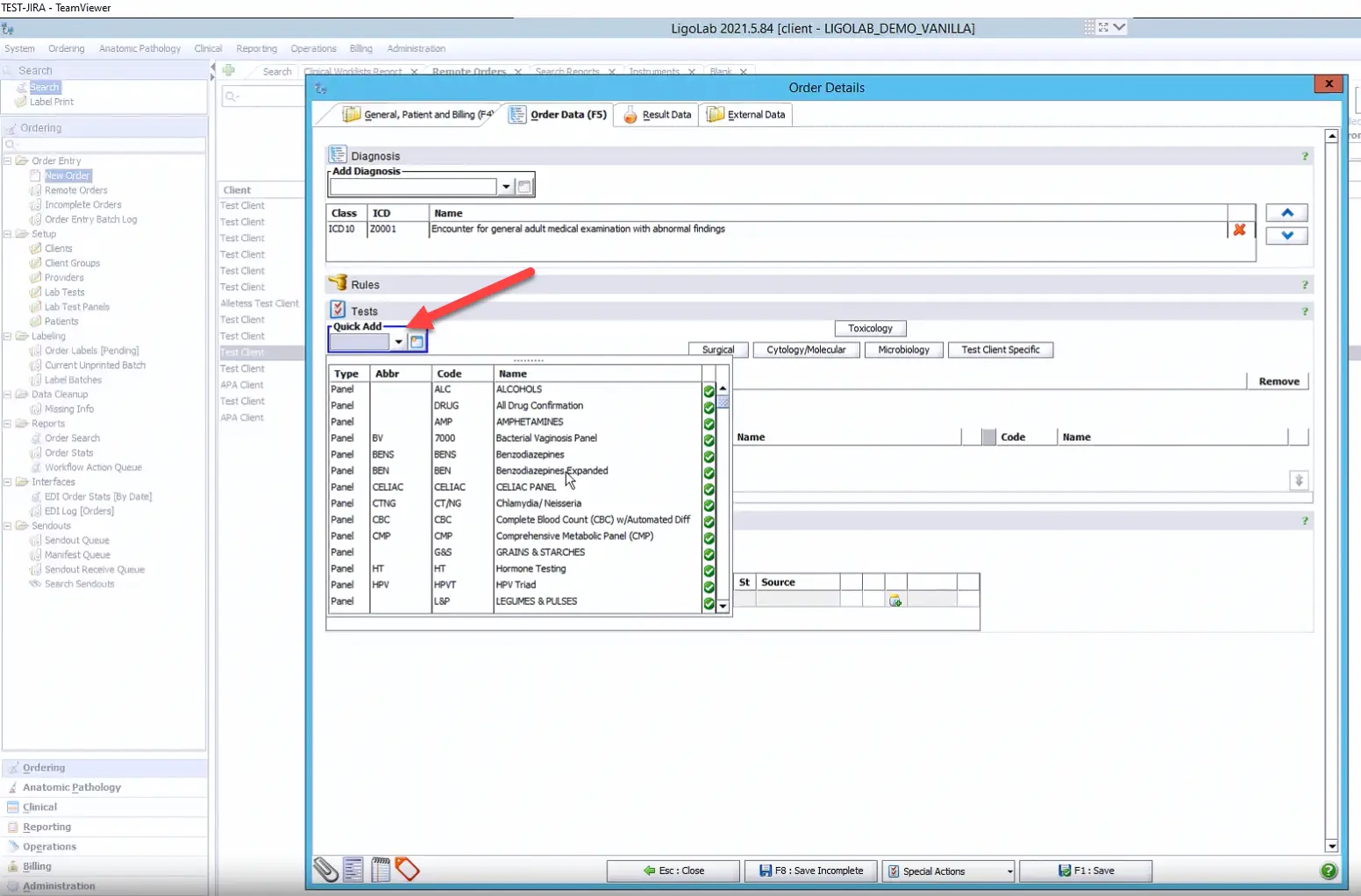
Adding ICD-10 Codes, Lab Tests, and Specimen Information in the Molecular LIS Module
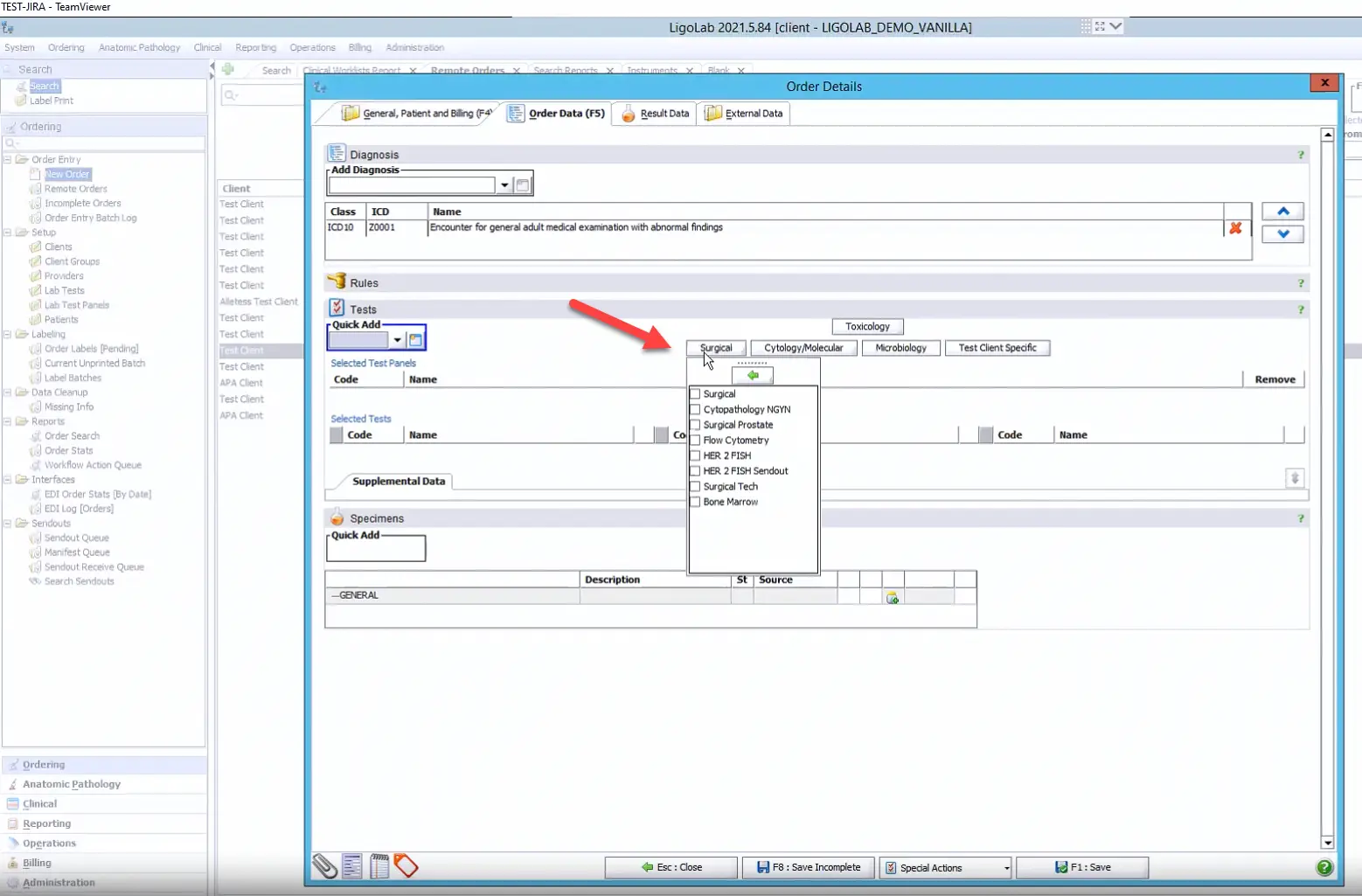
From here, the user goes to the Order Data tab in the Order Details window to add the ICD-10 codes, tests, and specimens. In the example below, the user has clicked on the Quick Add option to see a complete list of the lab’s tests and panels. Please note that this Quick Add functionality can be customized per client.
There is also a second, shortcut option for frequently ordered tests or panels. Once configured within the laboratory information system, these shortcuts save time for labs with large test menus.
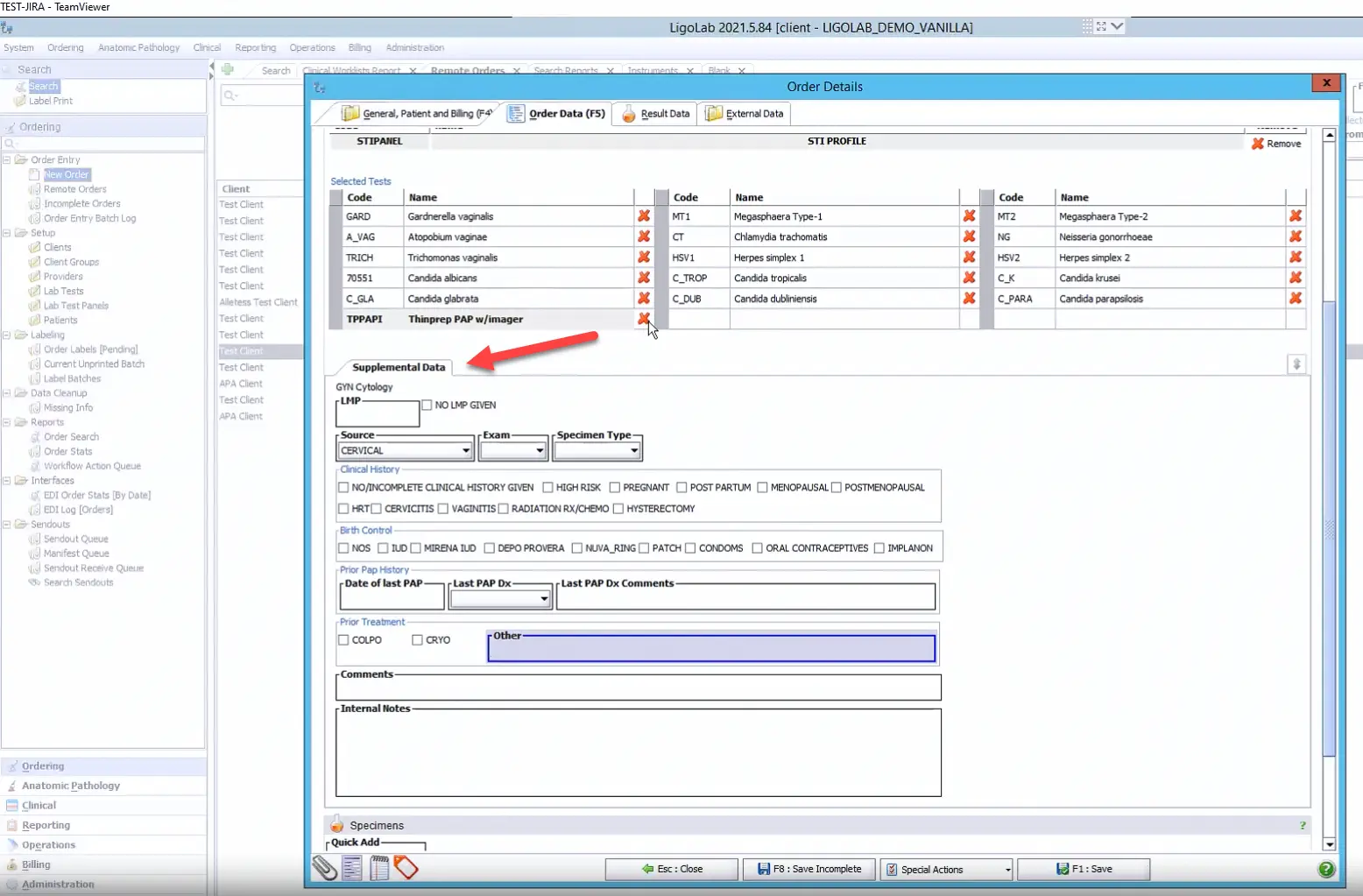
There is also the option to capture more data and add it to the order via the Supplemental Data fields. This area includes checkboxes and options for free text, and all of the info captured here is viewable by users downstream. The data can also be added to the lab report as needed.
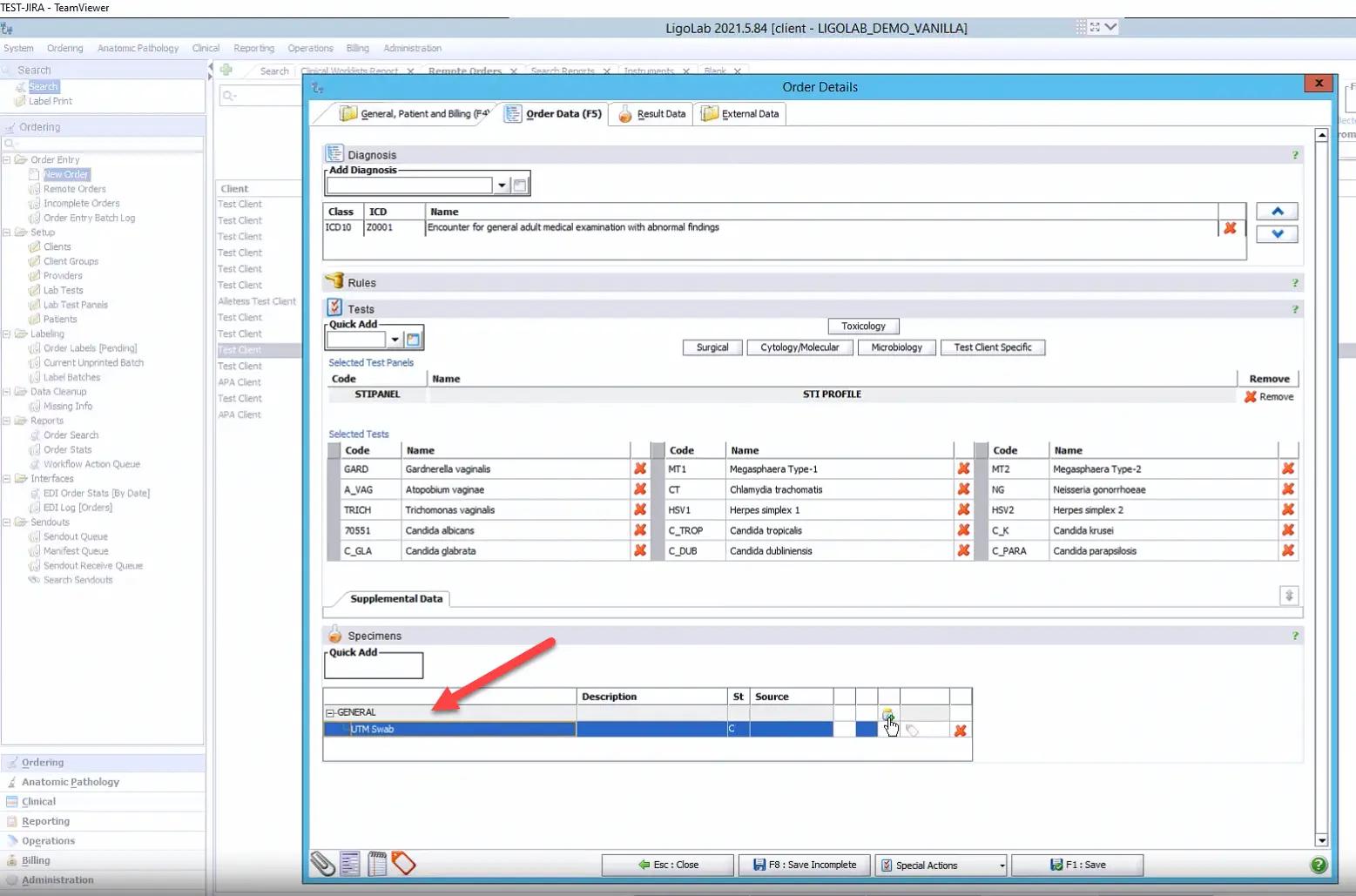
The final section in the Order Data tab is dedicated to specimen entry. It’s best practice to enter all specimens received with the case under a single order, unless they arrived as separate orders from the client’s EHR.
The molecular LIS module is built with intelligent logic that can anticipate workflow needs. For instance, it can automatically determine if a specimen should be split into aliquots for downstream processing. Once the order is saved, each specimen is assigned a unique identifier within the molecular LIS software database, ensuring accurate tracking throughout its lifecycle in the lab.
In the case highlighted below, a swab was automatically added to the Specimens section based on the combination of tests selected above it. Users can also add specimens manually, and each specimen has a built-in shortcut that can be typed into the Quick Add field in the Specimens section.
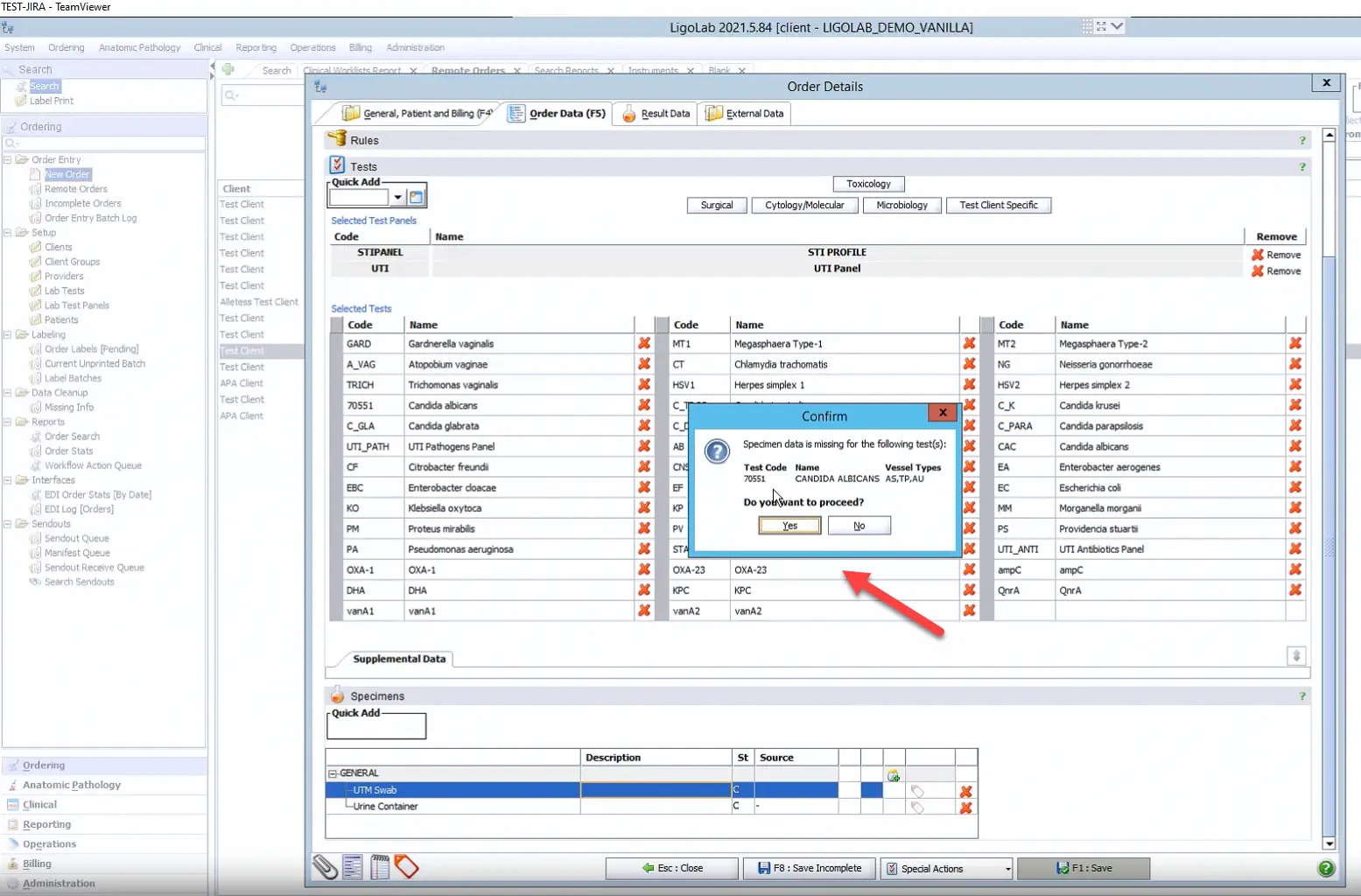
Next, the user clicks the Save button to save the order. If there are any issues with the order, the LIS software will send a notification and ask the user to confirm the order before proceeding.
What Happens in the Molecular LIS Module After the Order is Saved
Once the order is saved, an accession number is generated, and each specimen and test is assigned a unique identifier. Tests can then be grouped into reports based on the lab’s preferred workflow. For example, a lab may choose to combine all molecular tests into a single report or generate separate reports for UTI and STI results. Both configurations—and many others—are fully supported by the LIS software.
It’s also common for the LIS system’s automation to fire when the order is being saved. At this point, the molecular lab testing software will check to make sure that all relevant information has been included within the order (client, patient’s age, what testing was ordered, do other specimens need to be made, do subtests need to be added, etc.).
After the order is saved, a Label Print window automatically appears. It's recommended to print one label per specimen, with the specimen identifier embedded in the barcode. This ensures accurate, error-free tracking as each specimen is scanned throughout every stage of the clinical lab workflow.
Discover More: How Specimen Tracking Software Improves Efficiency and Reduces the Chance of Diagnostic Errors
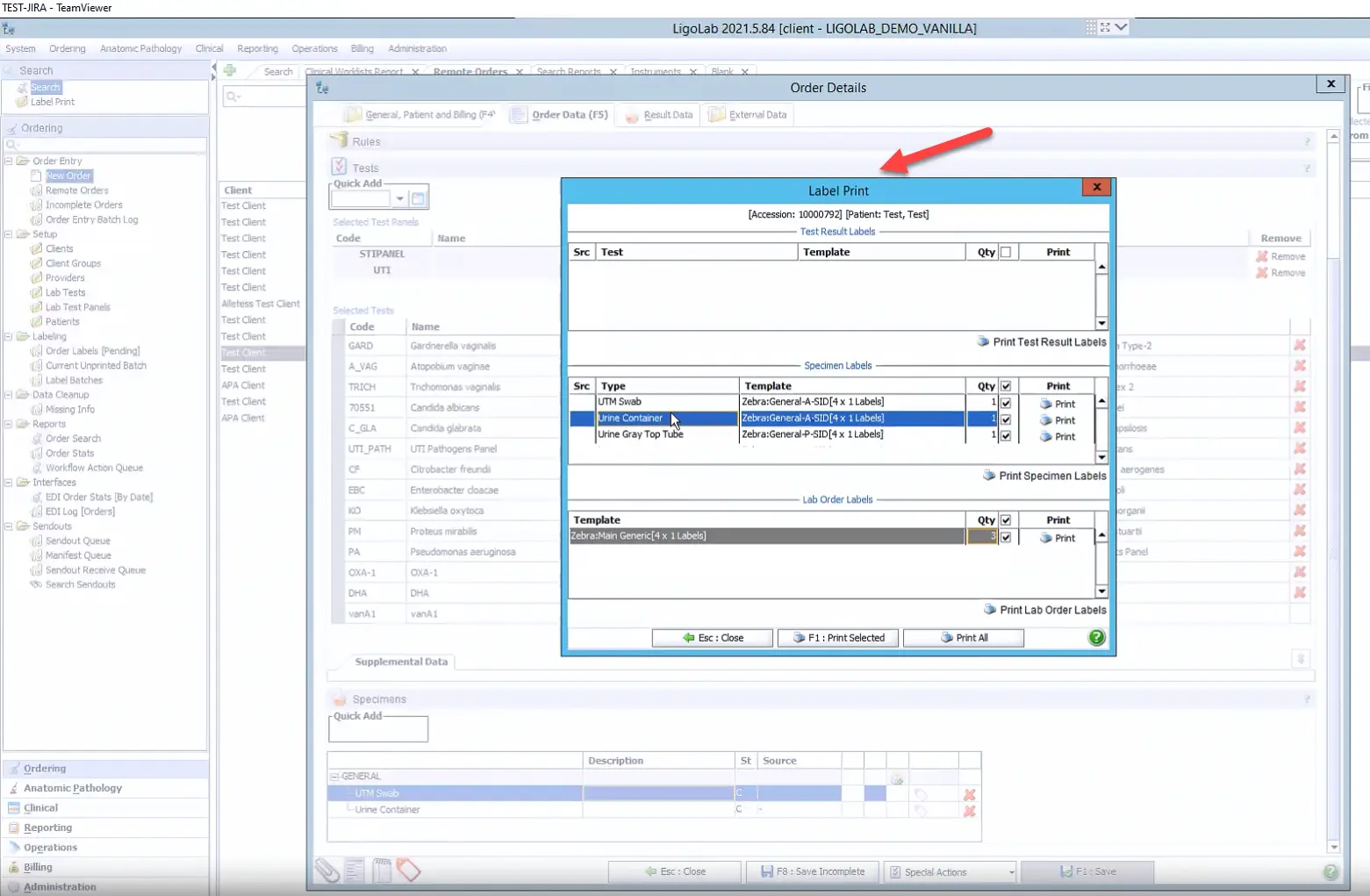
The lower section of the Label Print window (shown above) includes Lab Order Labels. For manually accessioned in-lab orders, we recommend labeling each page with a barcoded sticker. This allows documents—such as the original requisition, insurance details, and lab billing information—to be scanned in as Lab Order Attachments and easily accessed by any lab technician during specimen processing. Batch scanning these documents later in the workflow is also supported.
Once the Lab Order Attachments window is closed, the molecule LIS module is ready for the next order. The newly accessioned case and its scheduled tests automatically populate the appropriate workflow queues, signaling that processing can begin.
Learn More About LigoLab’s Molecular LIS Module
To discover how LigoLab’s molecular LIS module can elevate your lab’s performance—and why the LigoLab Informatics Platform is recognized as one of the best LIS systems available—simply fill out our request-a-demo form to schedule a quick introductory call with a LigoLab product specialist.
Book Your LIS Software Demo Today!







